-
Posts
2,500 -
Joined
-
Last visited
-
Days Won
2
Content Type
Profiles
Forums
Blogs
Events
Gallery
Everything posted by viking8x6
-
If, like me, you're prone to losing them - this is one possible solution: Buy silicone O-rings by the dozen. I got these from Amazon and they work out to a little more than $1 each. They're a bit on the narrow side, but they're fairly stiff while still stretchy enough to get on and off - work quite well for me. They are also available in a wide variety of sizes, which is a huge advantage. [think before following links] https://www.amazon.com/gp/product/B000FN0YLC/
-
I'm with @NWUSHorny on this one. I'm aware that this is a thing, but: I see people subscribing to this or that hierarchy, and jockeying for place in it, or happy with their place in it... and my reaction is very much "OK... whatever." I'm just not wired that way. I can role-play dom or sub (within limits) but it's definitely acting for me, and not at all connected with my psyche. I find politics similarly baffling.
-

Stopping PrEP: difference between Tops and Bottoms?
viking8x6 replied to hungry_hole's topic in PrEP Discussion
OK, a couple of points here. One, the 2-1-1 on-demand dosing for PrEP has been experimentally verified to be effective for bottoms. Therefore, a 2-day tail (plus however long it takes for the drug concentration to drop in the body) is sufficient to protect from infection. Therefore 28 days (or even 7) are not necessary. QED. Two [warning, science ahead!] the drugs (reverse transcriptase inhibitors) in PrEP do indeed go into the cells, and they must do so significantly *before* the HIV virus in order to protect that cell from infection. They must enter the cells because that is where the viral RNA is unpacked and transcribed into DNA - the first step in replication, and the one that RTIs prevent. They must do so early because the dosed forms of the drug are not the ones that inhibit the process - they must first be deprotected (from the chemical modification that protects them and allows them to be absorbed by the body) and then have a couple of phosphate groups added by body enzymes before they are in the form that binds to the viral enzyme and prevents it from working. All that takes some time. -
As many have already pointed out, "being at a sauna on [sic] a public sling" is NOT A SIMILAR SITUATION. "When you find you have gotten yourself into a hole, the first thing to do is stop digging."
-
I'm horny to some degree or another most of the time, but the level varies quite a bit. You could say I cease looking due to my lack of interest, but it would be more accurate to say that my interest isn't sufficient for me to believe that looking for sex is a good use of my time. Out here where I live (small town WV, more than half an hour from the nearest "decent size town" of over 10k people) it takes rather a lot of looking - or else a trip to a town or city - to get sex.
-
Firstly, I'd say you're NOT contributing to bugchasing. Your profile and posts make it clear that you aren't going to help people do it, nor to you condone or encourage it. Secondly, you are not responsible for the actions of others. That's on them. You happen to have HIV. Suppose for a moment that you did have a significant viral load (for whatever reason), and thus represented some kind of temptation to bugchasers who were serious about it, would not be a failure on your part. It would simply be a fact, whether or not you had made choices that caused it to be that way (either could be the case). The chaser's feelings and actions are their problem. The world is full of temptations and dangers... and dangerous temptations. It is not our responsibility to protect others from them, though it might be honorable and morally upright to do so, especially if they are less capable of doing so themselves (children, for example). Thirdly, and this is not intended to be judging you, merely to put things into perspective, we are living in a world where people hurt and kill themselves and others (not to mention wrecking the planet and other living creatures) all the damn time. Just now we have frequent mass shootings, pandemic, war, and global climate change. So concern over encouraging bugchasing merely by participating in BZ belongs (in my opinion) squarely in the category of "high-class worries".
-

What annoys you most about gay porn.
viking8x6 replied to Barebackpiggy's topic in Bareback Porn Discussion
Lousy audio: Horrible music (that's almost any of it, in porn), random background noise (sirens, machinery, you name it), can't hear the noises the participants are making... I could go on and on. Porn that's entirely (or almost entirely) closeup shots of dick-in-orifice, so you wouldn't know it's actually people fucking. Porn where the performers seem bored or mechanical, or the same fucking action continues unvaried for an extended period (3 minutes of pumping at the same speed and angle is more than plenty, gentlemen!) -
Ferocious applause - the latest is the best part yet! So much hotness! I especially loved the frat house scene - exhib is one of my hot buttons.
-
[think before following links] https://webarebears302.newtumbl.com/post/21877332-sg34Ay
-
You can only edit a post for about 10 minutes after you first post it. Thanks for reporting; I fixed it.
-
And afterward, it probably won't shrink back to its original size... even if you soak it in the great gray-green Limpopo river for ever so long!
-

How many guys on Breeding Zone have hooked up with each other?
viking8x6 replied to NWUSHorny's topic in General Discussion
One that I know of for sure, and I wouldn't be surprised if there were a few others at public venues (sex clubs mostly) over the years... in San Francisco and Denver mainly, but many other places from time to time. And if you ever do meet me out there and recognize me (the face pic is reasonably accurate) please do say something! It would definitely give the connection an added thrill. -
It looks to me like this is your point of conflict. You want to wear it... and by extension, you want to have a man fuck you bare and cum in you. But you don't want her to see that shirt. Ask yourself: Why not? You don't know what she would think, or how she would feel - she hasn't seen it yet! And yet she asked you to get tested. The implication of that is that she believes it is likely that you already have had a man fuck you bare and cum in you (or at least encountered that much risk of HIV exposure). And, from your observation, she's not freaked out by that, she's supportive. My advice (worth its weight in gold, remember!) is to get your hungry ass on PrEP and fulfill your needs... with your wife's blessing. And rejoice and be thankful that you are in such a wonderfully healthy relationship! And if you want that blessing explicitly ahead of time - there's only one way to get it (ask her). Just remember, neither you nor she knows for sure ahead of time how you will feel about it. You can guess, but the proof of the pudding is only in the eating.
-
I wouldn't even have thought to ask the question, let alone be so shockingly rude as to tell the host (before or after the event) that I wasn't attending or had issues with the other attendees because (to put it bluntly) I was racist. For a one-on-one encounter (or even a pre-planned small group), it seems reasonable to me that one's personal tastes or hot-buttons as far as sexual attractiveness would (and even should) be considered - no one wants to show up and have their trick go limp because they happen to be green, or have three eyes instead of two 😉 But at a group/party affair, it's unreasonable and inappropriate to expect that some attendees won't be your idea of the perfect man. After all, there are other people there who may well find those same people to be their cup of tea. I'm pretty sure this is not rocket science.
- 306 replies
-
- 4
-

-

-

-
- sex with latinos
- sex with black men
-
(and 1 more)
Tagged with:
-

"You are only allowed to send 0 messages per day"
viking8x6 replied to a topic in Tips, Tricks, Rules & Help
Until you delete them. There is a space limit, so eventually you may have to, but it's a pretty big limit. -
For what it's worth, this site isn't great for hookups, though it has more members in London than in most places. Not sure what hookup apps and sites are good over there across the pond. Here in the states, BBRT is good for bare, and Grindr and Sniffies seem to be fairly good generally. There's a topic in the General Discussion forum about what hookup apps people prefer.
-
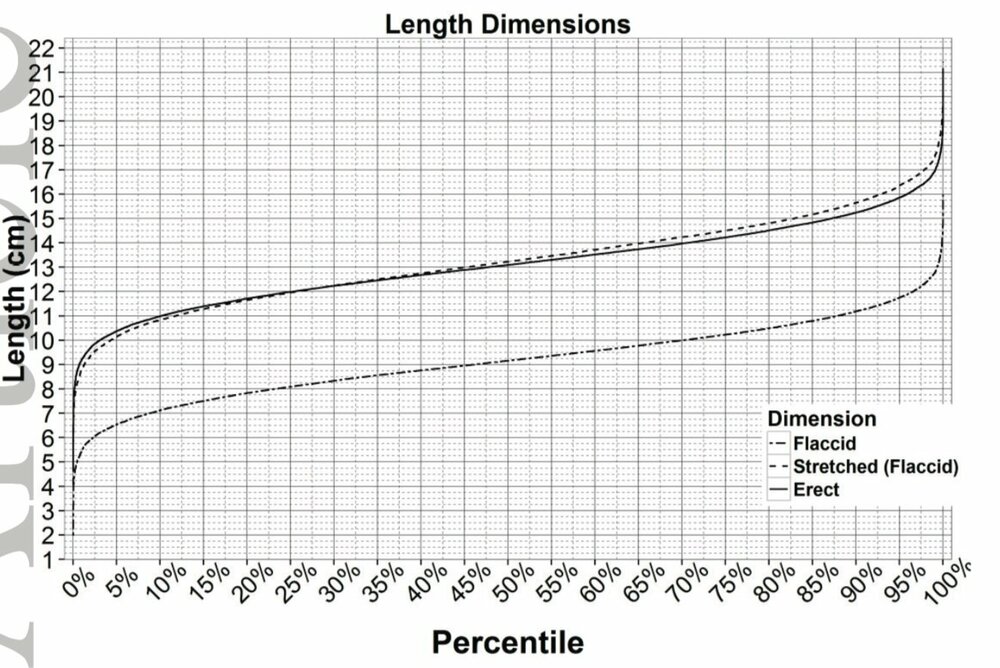
As a follow-up, here's a handy article about the subject: [think before following links] https://pubmed.ncbi.nlm.nih.gov/32666897/ And here's a nice chart (from this article: [think before following links] https://www.realclearscience.com/journal_club/2015/03/03/is_your_penis_normal_theres_a_chart_for_that_109106.html ) which tells us that over 99% of penises are 7 inches or smaller:
-
Average is actually a bit under 6, from the reading I've done. There's more than a little inflation and rounding up.
-
Well, as a true versatile, I have to say neither one feels better for me - they are simply different. I wind up topping more often than not, because tops are more in demand and because my endowment assures that it will be asked of me (why I don't know, big is good, but small is perfectly fun if the guy has a modicum of skill). Do what you enjoy! But being versatile doubles your chances of having a trick on a given night 🙂
-
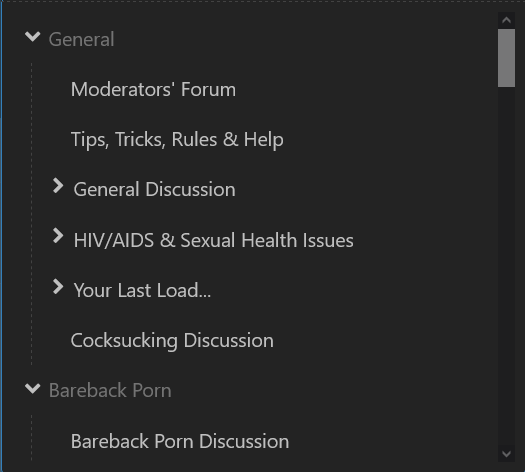
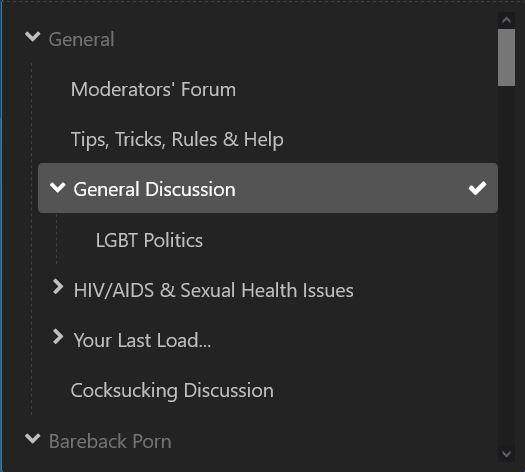
I'm gonna agree... but I'd rate it "mildly confusing" as UI glitches go. You simply click on "General Discussion" in the dropdown list. Yes, it's a header, but it's also a forum in its own right. That said, it's no doubt a feature of the (off-the-shelf) forum software and not of BZ in particular. Sadly, @rawTOP doesn't have control over these things. Happily, he's working on a new and better site! Before clicking (note how "General" is grayed out but "General Discussion" is not) After clicking
-

Guy Gets Bred By Father And Son
viking8x6 replied to rawTOP's topic in Bug Chasing & Gift Giving FICTION
Repaired. -
Wow, so hot! Just finished chapter 5 and it is quite delightful so far!
Other #BBBH Sites…
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.